Spleen size as a risk factor of cirrhosis and cirrhosis-associated symptoms
Liver cirrhosis is a severe and progredient disease frequently associated with episodes of decompensation (e.g. ascites, spontaneous bacterial peritonitis, upper gastrointestinal bleeding from varices and hepatic encephalopathy). Accurate and reliable prognostic tools to predict the clinical outcome of patients. and prevent deterioration need to be investigated.
Up to now various non-invasive and invasive prognostic risk factors associated with episodes of decompensation, need for liver transplantation or death due to hepatic decompensation are in clinical use (low platelet count, increased HVPG (hepatic venous pressure gradient, signs for oesophageal varices on endoscopy). CT/MRT measured spleen size and spleen volume might represent an elegant tool to predict morbidity and mortality in cirrhotic patients.
Project start: November 2012
Project status: clinical phase
Doctoral fellows: Julia Rjasanow
Tutor: Dr. med. Malinowski / Dr. med. univ. M. Jara |
Influence of Oxaliplatin based FOLFOX and CAPOX-Chemotherapy-Rigime on Liver function
New chemotherapy regime (e.g. FOLFOX, FOLFIRI, FOLFOXIRI) in combination with selective antibodies improved disease free and overall survival in patients with colorectal cancer. Moreover conversion chemotherapy turns former irresectable liver metastasis to resectable condition.
Recent studies stated hepatotoxic effects (i.d. steatosis, steatohepatitis or sinusoidal obstruction syndrome) deriving from preoperative chemotherapeutic treatment. Especially oxaliplatin-based regimes are directly linked to sinusoidal injury causing a spectrum of pathologies (sinussoidal damage; sinusoidal obstruction, peliosis or nodular regenerative hyperplasia) that are associated with increased perioperative morbidity. Thus it is from outstanding interest to display the actual liver function after adjuvant chemotherapeutic treatment before undergoing liver metastasis surgery.
Project start: September 2012
Project status: clinical phase
Doctoral fellows: Jan Bednarsch
Tutor: Dr. med. univ. M. Jara |
Fast track LiveR">Fast track LiveR - A prospective, multicenter study investigating the potential of LiMAx for risk stratification of patients after liver surgery
Project start: May 2012
Project status: clinical phas
|
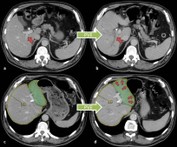 Prediction of partial liver function and volume after portal vein embolisation Prediction of partial liver function and volume after portal vein embolisation
Liver Resection is a standard procedure in patients suffering from liver metastasis or primary liver tumor. Up to 70-80% of the healthy liver volume can be removed without disadvantages to the patient. Due to regeneration processes liver volume and function reconstitute within 3 to 6 weeks after operation to preoperative values. However due to liver insufficiency, higher mortality rates occur in patients with low future liver remnant (<25%, FLR). Embolization of the right portal vein branch (PVE) is nowadays a commonly used procedure to pre-condition the liver before extended resection of the right lobe (liver segments IV-VIII). The volume of the left lateral liver lobe (FLR, segments II and III) increases due to PVE already before liver resection. Thus PVE leads to decreased postoperative morality and increased operability rates.
Project start: January 2012
Project status: clinical phase
Doctoral fellows: Thomas Keuchel / Clemens Wagner
Tutor: Dr. med. M. Malinowski
|
 a ICT-enabled, cellular artificial liver system incorporating personalized patient management and support a ICT-enabled, cellular artificial liver system incorporating personalized patient management and support
Chronic liver disease continues to be a frequent situation in today's population. Progressive liver tissue destruction results in inability to perform normal organ function. In turn, decompensation in chronic liver insufficiency results in impairment of liver function and development of complications. Liver failure is life-threatening and due to chronic graft shortage patients have to wait a long time for a curative organ replacement.
d-LIVER aims to provide remote ICT-enabled health monitoring of chronic liver failure patients in the home environment resulting in a much more efficient treatment and reduction of complications. Moreover the development of a remote monitored and controlled bio-artificial liver support devicer might replaced liver function properly. By means of the d-LIVER solution the quality of provided medical care may improve and in turn patients' quality of life might increase. Moreover d-LIVER might reduce the incidence and duration of hospitalization and consequently reduce the health economic burden of chronic liver disease.
Project start: November 2011
|
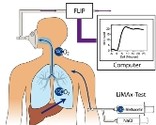 Early diagnosis of liver failure in septic patients using the LiMAx test. Comparison with conventional methods Early diagnosis of liver failure in septic patients using the LiMAx test. Comparison with conventional methods
Patients in a bacterial sepsis are on a high risk to develop a multi organ failure. Since the liver is highly vulnerable to a deficient perfusion, the occurence of sepsis will soon lead to a dysfunction of this crucial organ. During sepsis the mutual interaction between hepatocyts, kupffer cells and endothelial cells causes a very complex immune response. In this process the liver is not only a victim but it also keeps the inflammatory response syndrome a live.
Project start: July 2011
Project status: clinical phase
Doctoral fellows: Hannah Vetter / Navid Ahmadi
Tutor: Dr. med. M. Kaffarnik
|
 Influence of acute cholestasis on the liver function Influence of acute cholestasis on the liver function
Deterioration of liver function is frequently seen in patients suffering from chronic cholestasis. According to prior studies a negative effect can be observed in acute cholestatis in animals. Hence these findings need to be confirmed in clinical study on patients undergoing abdominal intervention.
Project start: January 2011
Project status: clinical phase
Doctoral fellow: Amir Kotobi
Tutor: Dr. med. J. Lock
|
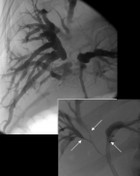 Clinical course of patients with Klatskin-tumour depending on operational intervention Clinical course of patients with Klatskin-tumour depending on operational intervention
Radical surgery represents the only curative option in patients with Klatskin tumors. Surgical intervention in terms of extended liver resection with en-bloc-resection of the extrahepatic bile duct can be either performed as an extended right-side hemihepatectomy or as extended left-side hemihepatectomy. Both interventions bear risk of postoperative complications. After extended right-side hemihepatectomy increased postoperative liver function due to larger resectate volume whereas after extended left-sided hemihepatectomy rather surgical complications such as bilioms have to be expected.
As there are no common criteria for decision making between extended right-side versus extended left-side hemihepatectomy in certain cases the decision which procedure will promise better results remains difficult.
Project start: January 2011
Project status: clinical phase
Doctoral fellow: Lina Demirel
Tutor: Dr. med. M. Malinowski
|
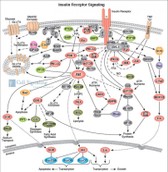 www.cellsignal.com www.cellsignal.com
Importance of expression patterns of several cytokines and growth factors after liver resection to estimate the potential of liver regeneration
Liver resection has become a standard therapy in modern surgery. Surgical procedures have been improved but the postoperative morbidity and mortality caused by liver dysfunction are still a major problem.
Therefore reliable und quick diagnostic tools in the field of liver function capacity are indispensable. The LiMAx test is a method to determine the maximum of liver function capacity. Aditionally there are surveys dealing with the temporal course of several cytocines and growth factors after liver resection. Most of these accomplished on animals show a correlation between cytocines/ growth factors and liver regeneration. Thereby there shoud be developed a better comprehension of the relationship between regeneration and function of the liver. In this process a better prediction of liver damage might be possible.
Project start: June 2010
Project status: clinical phase
Doctoral fellows: Juliane Aurich / Franziska Herrmann / Felix Wohlgemuth / Tim Reese
Tutor: Dr. med. A. Schulz
|
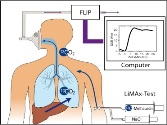 Influence of chemotherapy on liver function in patients with colorectal liver metastases Influence of chemotherapy on liver function in patients with colorectal liver metastases
Liver metastases are found in approximately 50% of the patients with colorectal cancer. Resection of these metastases is the only curative treatment for these patients. However, only about 20% of them are deemed resectable at the time of diagnosis of their metastases. Neoadjuvant chemotherapy has been found to improve resection rates and is therefore increasingly administered preoperatively in order to achieve higher resection rates and a better overall survival. However, recently concerns have arisen about the influence of chemotherapy on liver function and a resulting higher morbidity and mortality.
The objective of this study is therefore to investigate the influence of chemotherapy on liver function (measured with the new LiMAx-test) and to find a possible connection between chemotherapy, liver function, morbidity and mortality.
Project start: May 2009
Project status: writing
Doctoral fellow: Tilman Westphal
Tutor: Dr. med. J. Lock
|
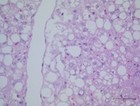 Transcriptional pathways of hepatic steatosis Transcriptional pathways of hepatic steatosis
Hepatic regulation of glucose and lipid homeostasis is influenced by various hormones, signalling pathways and transcriptional factors. Regarding animal-experimental research there is little doubt, that hepatic steatosis influences liver function and regeneration. An evidence for casual relationship between fatty liver and low grade of postoperative regeneration in human being is lacking. In our prospective clinical trial we will analyze human liver and fat tissue to provide datas concerning the molecular hepatic pathways in correlation to clinical liver function tests (LiMAx, ICG), blood parameters and a standardized clinical reporting form.
Project start: February 2009
Project status: writing
Doctoral fellow: Steffi Hoppe
Tutor: Dr. med. J. Lock
|
 Prognostic potential of different tests for liver function in patients with cirrhosis Prognostic potential of different tests for liver function in patients with cirrhosis
Despite an increasing demand of liver grafts for transplantation within recent years, the actual number of available donors has remained stable and insufficient. As a consequence, the patients' mortality on waiting lists remains a reasonable problem in medicine.
Therefore the allocation of grafts is a valuable tool to limit the problems arising from donor shortage. Effective transplant waiting lists allocate the available grafts firstly to those patients, who would have had a poor prognosis without transplantation. Nevertheless, it is a challenging issue to identify these patients, because a certain number of tests and classification systems are available to assess end-stage liver disease.
Liver allocation is currently based on the model for end-stage liver disease score (MELD). The MELD score includes 3 laboratory parameters: International Normalized Ratio (INR), serum creatinine and serum bilirubin. Clinical studies revealed its potential to predict short-term prognosis (6-month mortality). Nevertheless more and more authors criticize the problems and limitations of the MELD-Score.
Therefore additional parameters for the classification of graft function need to be explored for further optimization of the organ allocation system.
Project start: February 2009
Project status: clinical phase
Doctoral fellows: Susanne Biele / Dimitry Shelkov / Katja Lüttgert
Tutor: Dr. med. M. Malinowski / Dr. med. univ. M. Jara
|
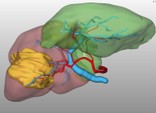 Enhancing the safety of oncological liver resection by virtual preoperative planning of the residual liver-volume and function (FUSION Study) Enhancing the safety of oncological liver resection by virtual preoperative planning of the residual liver-volume and function (FUSION Study)
In the current treatment of liver tumors, the curative approach of partial resection plays the most important role. Although this procedure is considered to be relatively safe, postoperative liver failure by inappropriate residual liver volume remains a life-threatening complication, which surgeons strive to avoid. Hence, a tool for the effective planning of postoperative liver volume and functional capacity is necessary to improve patients' outcome. We evaluate a novel system consisting of virtual resection by 3D-computer tomography and the LiMAx-Test for maximal enzymatic liver function capacity for enhanced planning of residual volume and function in a prospective clinical trial.
Project start: October 2008
Project status: clinical phase
Doctoral fellows: Rhea Röhl / Antje Kirchstein
Tutor: Dr. med. M. Malinowski
|
 Interpretation of non invasive breath tests- Improving the accuracy of the measurement method and development of new devices Interpretation of non invasive breath tests- Improving the accuracy of the measurement method and development of new devices
Non invasive breath tests provide valuable results on the liver function. In particular function can be determined by measuring the enzymatic product of 13C labeled substrates using a special analyzer. However online determination of 13CO2/12CO2 ratio is difficult and needs advanced detection techniques. In order to ensure further Sate-of-Art development of this measurement method Charité is cooperating with the Institute of Experimental Physics of the Free University of Berlin and a local company named Humedics.
Project start: 2007
Project status: In progress
Colaboration partners: Institute of Experimental Physics FU Berlin/ Humedics
|
| |








